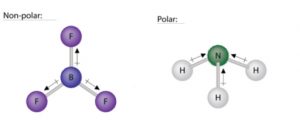
Ammonia (NH3) is a polar molecule while boron trifluoride (BF3), is a nonpolar molecule. What is the difference in the polarity of these compounds? - Quora
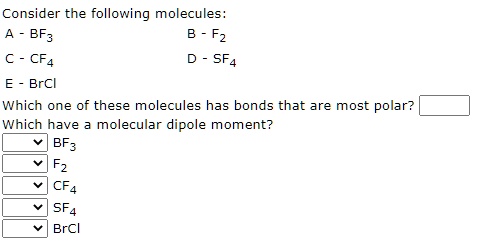
SOLVED: Consider the following molecules: BF3, B - F2, C - CF4, D - SF4, BrCl. Which one of these molecules has bonds that are most polar? Which have a molecular dipole

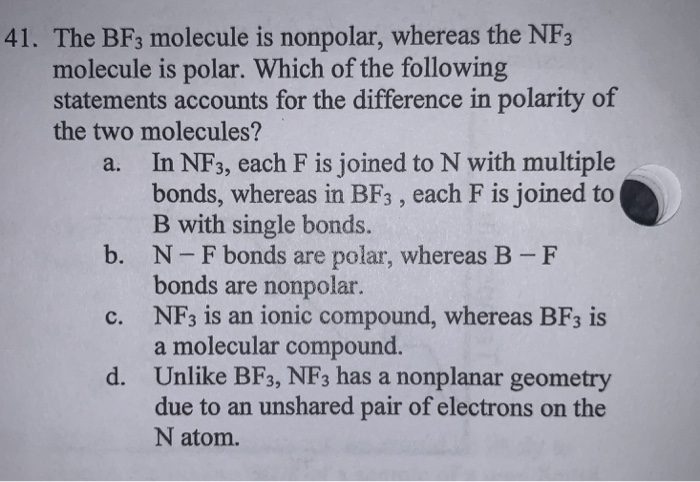
The molecule BF3 and NF3 , both are covalent compounds but BF3 is non - polar and NF3 is polar. The reason is that:

Which of the following is a false statement about BF3? A. BF3 has trigonal planar molecular geometry. B. BF3 has trigonal pyramidal electronic geometry. C. All three bond angles in BF3 are

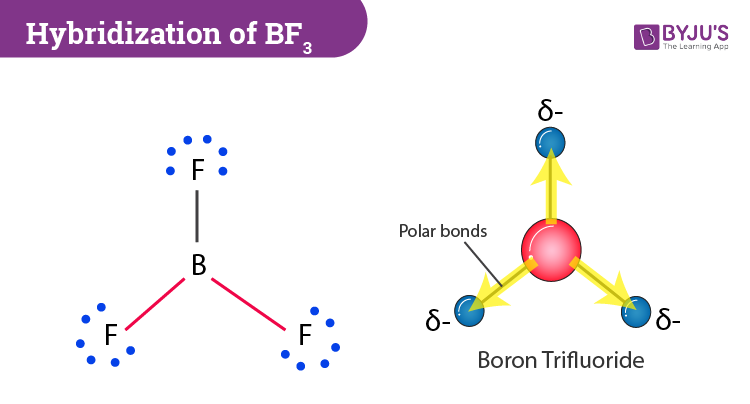
BF3 Molecular Geometry, Shape and Bond Angles (Boron Trifluoride) | Molecular geometry, Molecular, Geometry








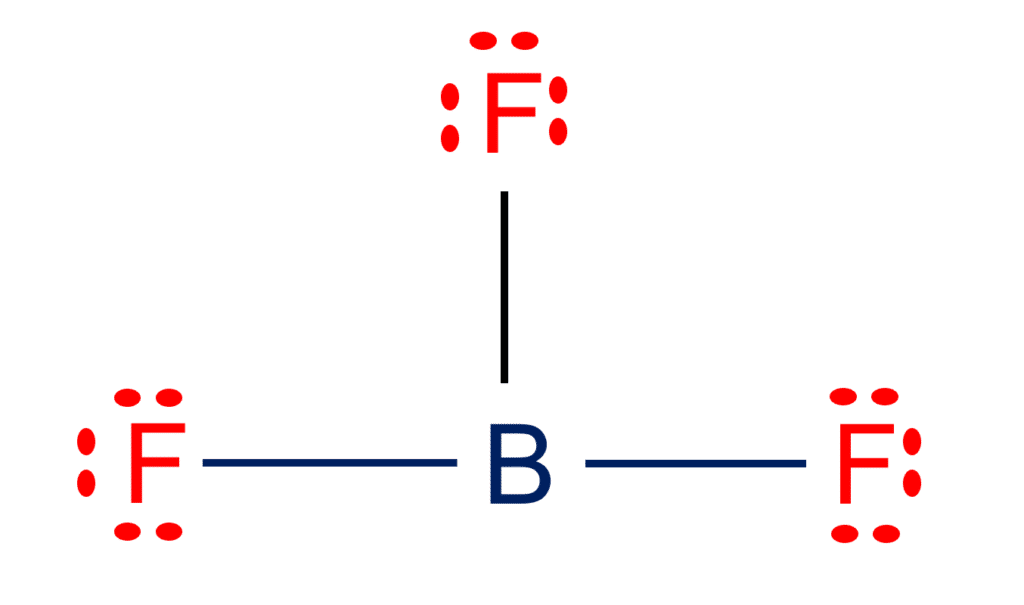
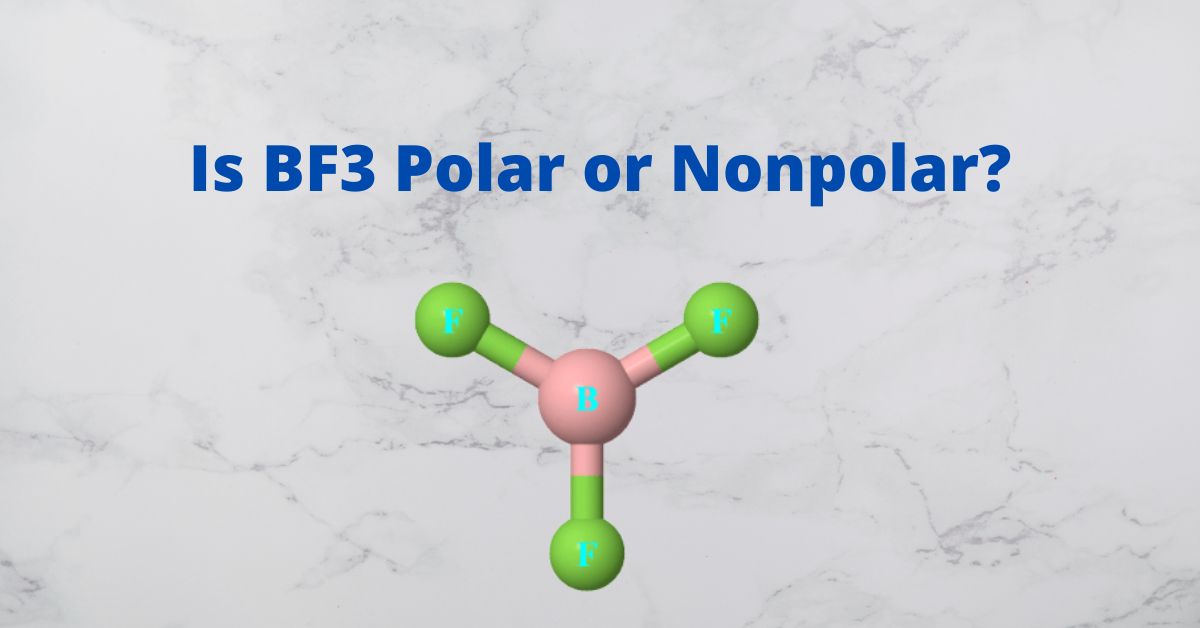
![Best Overview on: BF3 Polar or Nonpolar [#1] - Science Education and Tutorials Best Overview on: BF3 Polar or Nonpolar [#1] - Science Education and Tutorials](http://sciedutut.com/wp-content/uploads/2021/05/bf3-1.png)