
Polarity‐Reversed Allylations of Aldehydes, Ketones, and Imines Enabled by Hantzsch Ester in Photoredox Catalysis - Qi - 2016 - Angewandte Chemie - Wiley Online Library

Acetato de etilo, molécula de etanoato de etilo. Es éster de acetato, solvente aprótico polar, aditivo adicional E1504. Modelo molecular. 3D renderizado. Illutr Fotografía de stock - Alamy

Are All Polar Molecules Hydrophilic? Hydration Numbers of Ketones and Esters in Aqueous Solution | The Journal of Physical Chemistry B

HSP solvent polarity map showing all permutations of esters with T b... | Download Scientific Diagram

A chemoselective polarity-mismatched photocatalytic C(sp3)–C(sp2) cross-coupling enabled by synergistic boron activation | Catalysis | ChemRxiv | Cambridge Open Engage

Relative reactivity and polarity of carboxylic acid derivatives. X = halide | Download Scientific Diagram

ESTERS. What is an ester? Responsible for tastes and odours. Contain the ester linkage Made from an alcohol and a carboxylic acid. Since the C=O group. - ppt download
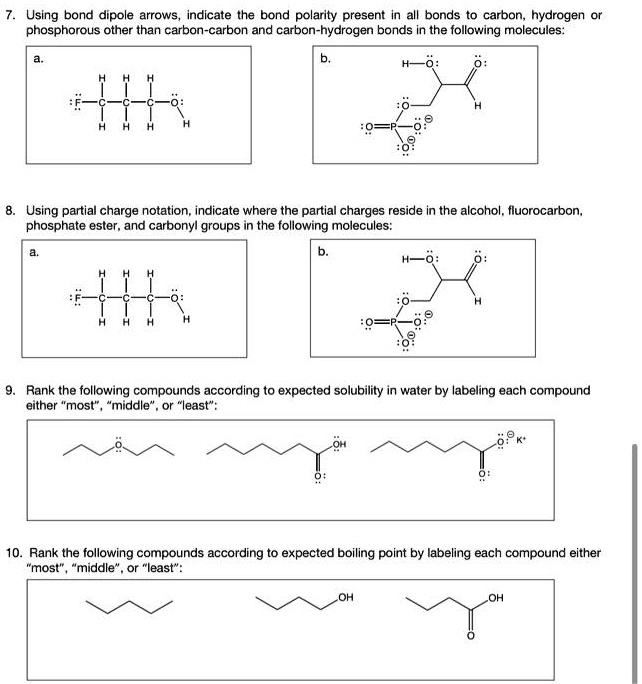

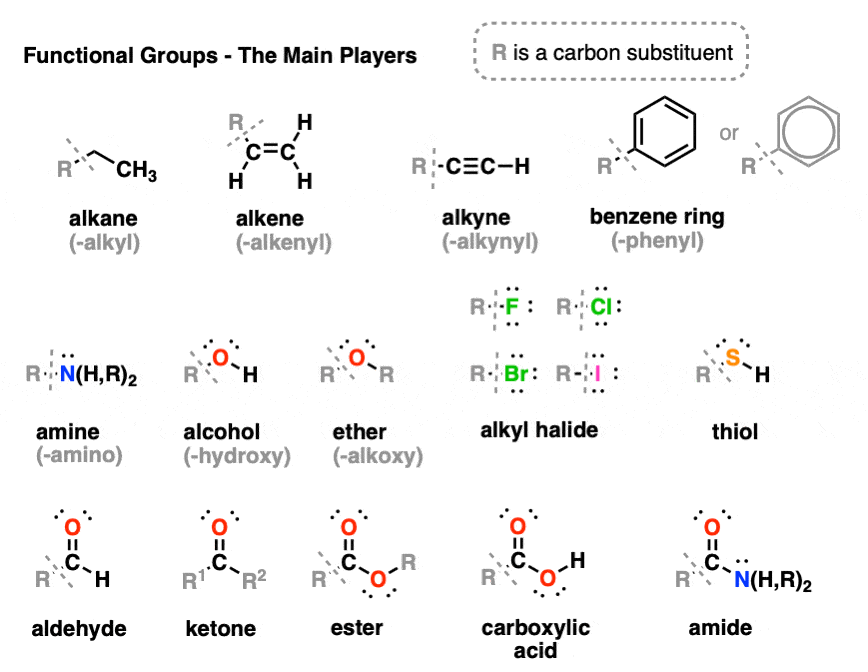

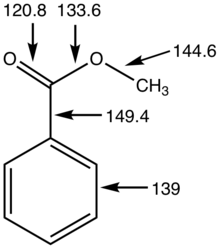


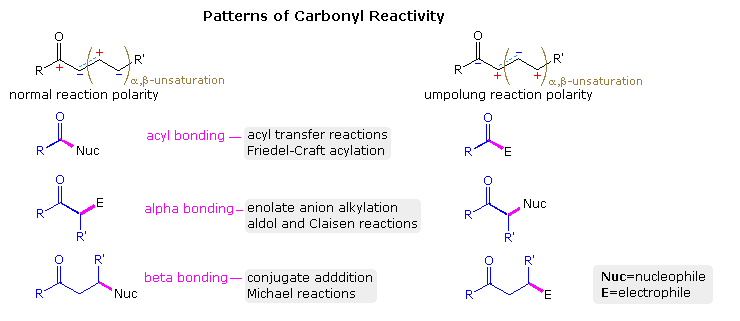


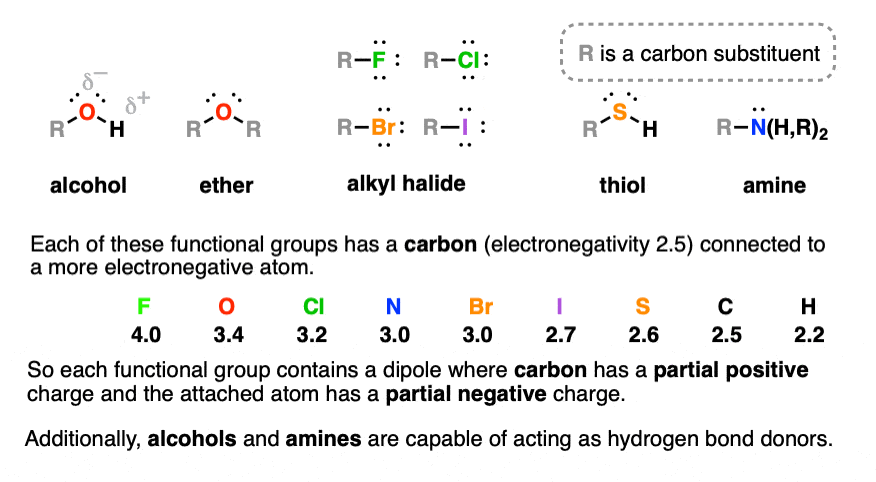


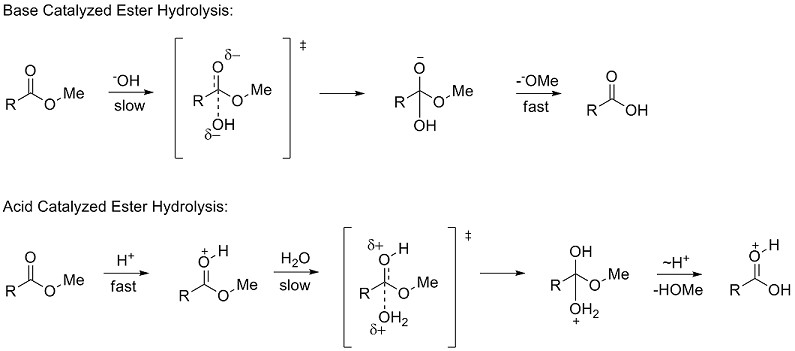

:max_bytes(150000):strip_icc()/ester-59134cd83df78c9283519859.png)


